Just floating a
back-of-envelope calculation about 12C:13C
ratios in different parts of what I am going to call the 'dynamic'
carbon cycle: the bit where carbon is moving around a lot, leaving
out both the slow deposition of fossil fuels and carbonates and the
geological or anthropogenic processes that get them out of the ground
again.
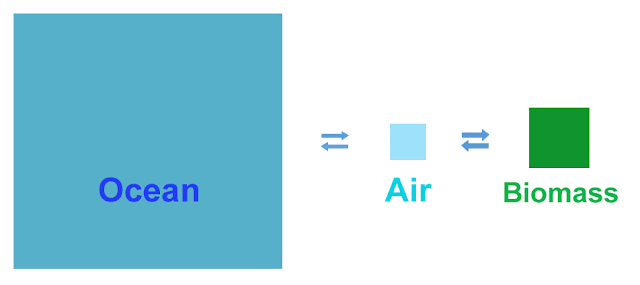
I've taken some numbers
from one of many pictures of the carbon cycle available on Our Friendthe Interwebz, and shown them to scale
the way the ancient Greeks would have, geometrically. The area of the
arrows and the squares are to the same scale, showing the amount of
carbon in each place and the amount moving from one place to another in the
course of a year. There are three populations of carbon in this
simplified picture: Biomass (plants and soil), the atmosphere, and
the oceans. And the greatest of these is the ocean.
Note that we basically
have a fast equilibrium (the atmosphere and biomass equilibrating
rapidly, on the rough order of a decade to exchange half the carbon
in the biomass) and a slow equilibrium (the atmosphere and the ocean
doing the same on the order of a few hundred years).
Before moving on, I
just want to say that the overall ratio of 12C:13C
in this whole system should remain pretty much the same for periods
of geological time that are long compared the time scales in which
lots of interesting things can happen. Good evidence for this is that
the amount of variation in carbonates laid down since the beginning
of the Pliocene is not very much at all: less than +/- 1 ‰
(Ghosh & Brand 2003).
Now, the relative
amount of 12C:13C in each box will be governed
by two things:
(1) The relative
amounts of 12C:13C in the box(es) it is in
equilibrium with; and,
(2) Any isotopic
selectivity in the transitions between boxes.
Now, the isotopic
selectivity due to the more rapid diffusion of 44CO2
over 45CO2 through eensy-weensy membranes in plants is very
well understood: and this generates a clear difference in the 12C:13C
ratio between biomass and the atmosphere, despite the rapid
interchange between them.
But, if there was no
life on Earth, the atmosphere would still be enriched in 12C:13C
relative to the ocean. Because there is also a solid basis for
isotopic selectivity in the ocean:atmosphere equilibrium. Most of the
carbon in the ocean is present as hydrogen carbonate ions in the deep
ocean. To get to the surface, this material has to run a gauntlet of
a layer of warm water hundreds of metres thick where calcium
carbonate is stable. I haven't been able to find any decent data on
isotopic dependence of hydrogen carbonate ion diffusion rates - just
some molecular dynamics simulations that didn't find a significant
difference* - but a priori, if you have a column of fluid some
hundreds of metres high, surely the bottom of the column is going to
be enriched in the component with a molar mass of 62 rather than 61.
Now it seems to be the
because of the fast interchange between the biomass and the
atmosphere, the relative distribution of carbon isotopes between the
ocean and the total (biomass + atmosphere) component has to be
governed by the slow equilibrium. And thus the main driver of the
isotopic ratio in the atmosphere has to be the slow equilibrium. Yes?
If you see a problem with this, let me know.
So: looking back at
those boxes. And going back to that number from Ghosh & Brand
2003 and some other numbers. Taking
-6 13C
‰
for the pre-industrial recent atmosphere and -25 13C
‰
for terrestrial biomass (Epstein 1969) gives an overall value of -20
13C
‰
for the (biomass + atmosphere) component. Now, if we have a 13C
‰
of 0 ±
0.8 for oceanic carbonates since the Pliocene, this means that the
error we should reasonably associate with this
component should be of
order ±
10 13C
‰.
This
may be a conservative overestimate: but looking at the size of the
boxes where the carbon is sitting, the error in the small boxes has
to be bigger than the error in the small boxes. And there is one
more small problem, which is that while the 12C:13C
selectivity in plants is well-understood and not likely to have
changed much at all since the little fellers evolved their carbon
fixation pathways, the 12C:13C selectivity of
ocean:atmosphere transport ought to be dependent on the distribution
of the layer of warm water, which will rely on the vicissitudes of
global climate and over a longer period of time how the continents
are shifting about to change global ocean circulation.
So,
my point is: relying on 12C:13C ratios alone
to tell us details about the source of a particular carbon-containing
deposit at some remote period of time is probably not a good idea, because there are likely to be big systematic errors which we know not what of.
Perhaps
that's not a very big point.
* And only just found a
significant difference for CO2, where we know one exists
experimentally.
No comments:
Post a Comment